Blogue
07
Jan
2019
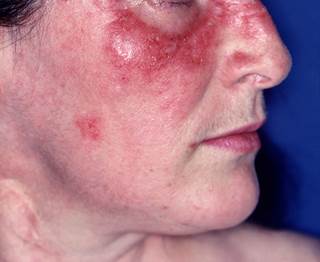
B-Cell Esgotamento terapia com rituximab eficaz para Lúpus eritematoso cutâneo