Blogue
28
dezembro
2018
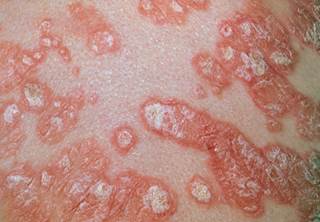
Long-Term Ixekizumab Tratamento Aceitavelmente Seguro na psoríase em placas